Are we prescribing antibiotics wrong?

Outsourcing Clinical Trials Outside of the US

Regulators And Standards Groups Take Steps To Address Emerging Technologies In Biopharma

Two-dimensional liquid chromatography with multiple heart-cutting
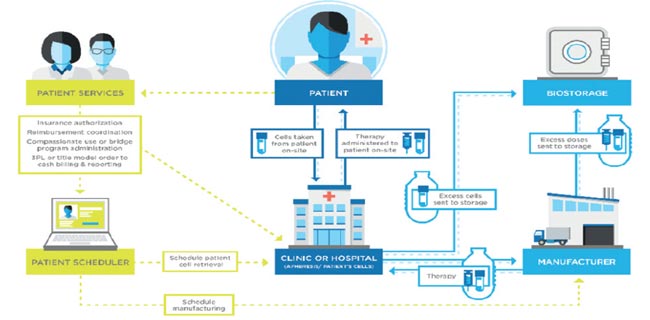
Understanding Pharmaceutical Logistics Validation in a Dynamic Environment





Latest White Papers
- Random
Featured News
Latest News
- Title
- Most Views
- Recently Added
- Ordering
- Random



Biohaven Ltd. a global clinical-stage biopharmaceutical company focused on the discovery, ...

AptarGroup, Inc. a global leader in drug delivery and consumer product dosing, dispensing and ...

Virax Biolabs Group Limited an innovative biotechnology company dedicated to the advancement of ...

AstraZeneca’s IMFINZI® in combination with standard-of-care FLOT chemotherapy (fluorouracil, ...



Latest Updates
PICO IV, a leading innovator in sterile cannabidiol (CBD) solutions, announced a closely coordinated clinical collaboration with BrainThrive, PC, a neurology and diagnostic testing center in Westlake ...
PICO IV, a leading innovator in sterile cannabidiol (CBD) solutions, announced a closely coordinated clinical collaboration with BrainThrive, PC, a neurology and diagnostic testing center in Westlake Village, California.
The partnership places PICO IV's patented sterile CBD solution within a ...
AptarGroup, Inc. a global leader in drug delivery and consumer product dosing, dispensing and protection technologies, announced that its innovative nasal vaccine delivery solutions, LuerVax® and ...
AptarGroup, Inc. a global leader in drug delivery and consumer product dosing, dispensing and protection technologies, announced that its innovative nasal vaccine delivery solutions, LuerVax® and Spray Divider™, are being utilized in CastleVax’s Phase II clinical trial of CVAX-01, a next-generation ...
Fabentech, a French biopharmaceutical company specializing in medical countermeasures against biological threats, announces that it has been granted Marketing Authorization for Ricimed®, a treatment ...
Fabentech, a French biopharmaceutical company specializing in medical countermeasures against biological threats, announces that it has been granted Marketing Authorization for Ricimed®, a treatment for ricin poisoning.
Ricimed®, an antidote against one of the most toxic natural substances in ...
Dechra, a global leader in veterinary specialty care, today shares the FDA approval of Emeprev™ (maropitant citrate) Injectable Solution, the first FDA-approved bioequivalent injectable solution to ...
Dechra, a global leader in veterinary specialty care, today shares the FDA approval of Emeprev™ (maropitant citrate) Injectable Solution, the first FDA-approved bioequivalent injectable solution to the most widely used antiemetic for dogs and cats.
Indicated for the prevention and treatment of ...
BD a leading global medical technology company, announced the global commercial release of new configurations of cell analyzers featuring breakthrough spectral and real-time cell imaging ...
BD a leading global medical technology company, announced the global commercial release of new configurations of cell analyzers featuring breakthrough spectral and real-time cell imaging technologies, enabling more labs in academia, pharma and biotech – across scales, needs and budgets – to advance ...
Upsher-Smith Laboratories, LLC announced the launch of KYMBEE™ (deflazacort) Tablets, indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients 5 years of age and older. ...
Upsher-Smith Laboratories, LLC announced the launch of KYMBEE™ (deflazacort) Tablets, indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients 5 years of age and older. Manufactured by Upsher-Smith in Minnesota, KYMBEE™ is the only deflazacort backed by Upsher-Smith's Promise ...