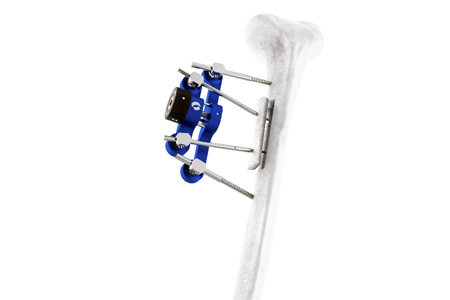
Orthofix Medical Inc. a leading global medical technology company, announced it has received U.S. Food and Drug Administration (FDA) 510(k) clearance and the European CE Mark for the TrueLok™ Elevate Transverse Bone Transport (TBT) System.
TrueLok Elevate provides a limb preservation treatment option for addressing bony or soft tissue deformities and defects such as diabetic foot ulcers and nonhealing or deep tissue wounds. While the TBT procedure has previously been reported,1 TrueLok Elevate is the first dedicated system for TBT to receive FDA clearance. The product is currently available in a limited market release in the U.S. and select international markets.
“Transverse bone transport with TrueLok Elevate now enables surgeons to effectively address challenging conditions in a patient’s extremity by allowing for an efficient and reproducible method to create a bone segment in the tibia that can then be gradually distracted over a period of several days,” said Dr. Emmy Oji, a podiatric foot and ankle surgeon at Valley Foot and Ankle Specialty Providers, who was among the first surgeons to apply the TrueLok Elevate system to a patient. “Clinical publications have shown this approach improves blood circulation to the affected limb and promotes wound healing in diabetic foot, potentially reducing the need for amputation.” Additionally, first patient cases in Europe were performed in the UK and Germany.
According to the American Diabetes Association, over 160,000 amputations occur per year in the U.S. as a result of diabetic-related complications, representing a sizable market opportunity of approximately $1.2 billion. In addition, published studies have shown that patients with diabetic foot ulcers who receive an amputation have a five-year mortality rate of 57% and are burdened with lifetime healthcare costs of just over $640,000 for care directly related to their amputation.2,3 Therefore, TrueLok Elevate offers the potential to not only be a limb and cost-saving device, but most importantly, a life-saving solution to a challenging patient population.
“The introduction of the TrueLok Elevate system is a pivotal milestone in demonstrating our commitment to leading growth in the limb reconstruction market,” said Patrick Fisher, President of Global Orthopedics for Orthofix. “Within our orthopedics business, we are focusing on providing innovative solutions that spans four pillars: Limb Preservation, Extremity Deformity Correction, Limb Lengthening, and Complex Fracture Management to aid surgeons in managing patients with complex limb reconstruction needs.”
Those attending the American College of Foot & Ankle Surgeons (ACFAS) Annual Scientific Conference in Phoenix, AZ from March 27-30 can learn more about the TrueLok Elevate System by visiting booth 1045.
The TrueLok Elevate system represents the latest addition to Orthofix’s flagship product line, the TrueLok family of multiplanar external fixators, including the TrueLok EVO and TL-HEX™ ring fixation systems. For indications, contraindications, warnings, precautions, potential adverse effects, and patient counseling information see the Instructions for Use or contact your local representative.
For more information please visit www.Orthofix.com