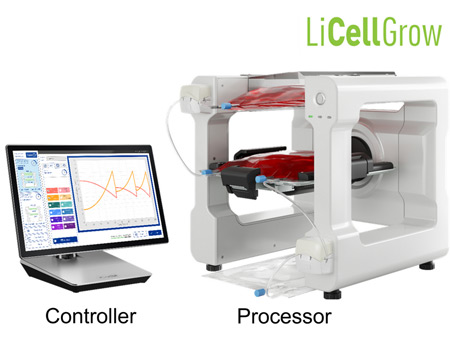
PHC Corporation Biomedical Division will showcase a prototype of its new cell expansion system LiCellGrow at the ISCT Regional Meeting. LiCellGrow, currently under development, is designed to allow pharmaceutical companies to visualize continuous metabolic changes in cells in real-time and adjust the cell culture automatically to optimize conditions for cell growth. The system seeks to expand availability of cell and gene therapy (CGT) products by accelerating the manufacturing of specific cells needed for these therapies.
CGT, in which a patient’s cells are removed, modified, and reintroduced to the body to fight disease, has emerged as a promising therapeutic field for difficult-to-treat conditions such as cancers and hematologic disorders. Since the patient’s own cells are used as raw materials, it is essential to maintain stable cell quality when producing new cells, to counteract natural variations in cell characteristics and ensure consistency throughout the therapy manufacturing process. The current method of evaluating cell quality by sampling the final product may lower both manufacturing efficiency and yield, increasing production cost.
LiCellGrow seeks to enable more efficient and stable manufacture of high-quality CGT products by enabling researchers to monitor and control the cell culture environment in real time, optimizing cell quality while minimizing interruption to the therapy manufacturing process.
The system under development uses proprietary In-Line monitoring technology, which continuously measures cell metabolites in the culture medium without the need for repeated sampling by immersing sensors in the medium at all times. LiCellGrow then continuously and accurately measures concentrations of two critical indicators of cell metabolism, glucose and lactate, to offer a visualization of actual changes in the culture environment and cell status. Based on real-time measurement data, the system then automatically exchanges the culture medium to maintain the culture environment in an optimal state. This technology is designed to improve and homogenize cell quality while increasing cell culture efficiency and reducing costs through loss reduction. The system will also feature easy-to-install single-use culture bags with In-Line sensors, enabling closed-system cell culturing to maintain a sterile environment, and can be installed in existing CO2 incubators, enabling researchers to conduct studies using their preferred equipment.
LiCellGrow is an example of synergy between the Biomedical Division and IVD Division (hereinafter referred to as “IVD”) of PHC Corporation. The In-Line monitoring technology featured in this cell expansion system is newly developed proprietary technology building on the core technology of blood glucose sensor, the main product developed by IVD. By combining this sensor technology from IVD with advanced cell culture environment control technology from the Biomedical Division, PHC seeks to offer researchers and pharmaceutical companies greater value.
The Biomedical Division plans to launch LiCellGrow to support the development of manufacturing processes for CGT products globally in the short term, and aims to accelerate development of this system to ultimately support commercialized production of CGT products.
LiCellGrow builds on the Biomedical Division’s 20-year history of pioneering solutions in the field of cell therapy, including the Cell Processing Center*1 in 2000 and the Cell Processing Isolator *2 in 2007. Moving forward, we will continue to accelerate the creation of innovative solutions addressing QCD (Quality, Cost, Delivery) challenges in the therapeutic manufacturing process for early adoption of CGT, aiming to contribute to advancing modalities (therapy methods.)
About the Biomedical Division of PHC Corporation:
Established in 1969, PHC Corporation is a Japanese subsidiary of PHC Holdings Corporation (TOKYO:6523), a global healthcare company that develops, manufactures, sells, and services solutions across diabetes management, healthcare solutions, life sciences and diagnostics. The Biomedical Division supports the life sciences industry helping researchers and healthcare providers in around 110 countries and regions through its laboratory and equipment and services including CO2 incubators and ultra-low temperature freezers.
For more infrmation please visit, www.phchd.com