MLB (Merry Life Biomedical Company, Ltd., a biomedical company, announced that the U.S. Food and Drug Administration (FDA) has approved IND application for TML-6, an novel drug to treat Alzheimer's disease (AD), enabling a Phase 1 clinical trial to be initiated this July. Advancing TML-6 into clinical trial is a critical milestone for MLB to develop a new era multi-target drug for AD since 2018.
About TML-6:
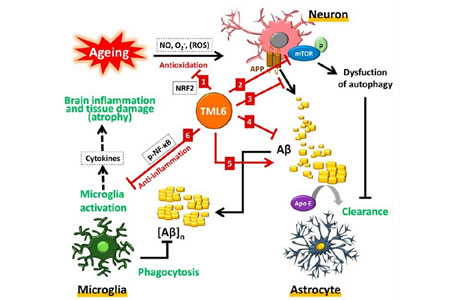
TML-6 is a novel synthetic curcumin analog. Professor Ih-Jen Su at Southern Taiwan University used a platform of 6 aging and AD biomarkers to screen the 12 compounds from Androscience (San Diego, USA) for AD candidate. Preclinical studies showed that TML-6 (ASC-6) exhibits a multi-target action mechanism for AD, including anti-aging, activation of autophagy (mTOR inhibitor), reducing amyloid accumulation, and anti-inflammation (Figure 1), the efficacy confirmed by 2 AD animal models. TML-6 has a high bioavailability through formulation and then completed preclinical toxicology and safety studies. TML-6 should be potentially a novel drug to improve or reverse the progression of early-stage AD.
The Design and Future Plan of TML-6 for AD Clinical Trials:
TML-6 is developed as an oral drug and will conduct a SAD/MAD phase 1 clinical trial at Glendale Adventist Medical Center, LA, USA in 2024 Q3. Elderly cohort and CSF pharmacokinetics (PK) studies were specifically designed. For a global multi-site phase 2a clinical trial, several distinguished global AD experts provided consultation. Blood biomarkers will be included in the phase 2a trial as the surrogate endpoint of efficacy. Furthermore, TML-6 is considering to combine with the current anti-amyloid drugs in phase 2 trial. The drug combination could not only exhibit synergistic effects to improve AD behavior, reducing amyloid accumulation and ant-inflammation, but can also reduce antibody dosing to only 10% and avoid the adverse events (ARIAs) of anti-body drugs. MLB has successfully raised funds to conduct this global phase 2a clinical trial, scheduled to be conducted on 2025 Q3.