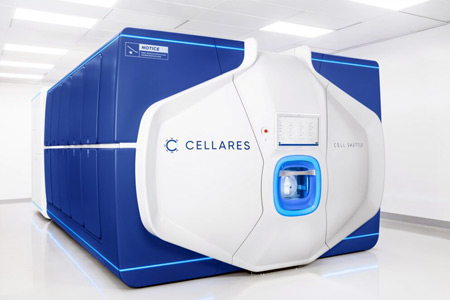
Cellares, the first Integrated Development and Manufacturing Organization (IDMO) dedicated to clinical and commercial-scale cell therapy manufacturing, announced the commissioning of the first cGMP Cell Shuttle™ at the IDMO Smart Factory in Bridgewater, New Jersey. This marks an important milestone on the way to a fully fitted facility, which will be capable of producing up to 40,000 standard CAR-T cell therapy doses per year, or up to 100,000 doses of novel, two-day process CAR-T cell therapies.
The Bridgewater, NJ IDMO Smart Factory is a clinical and commercial-scale cell therapy manufacturing innovation. The facility integrates advanced, end-to-end automation technologies including the Cell Shuttle and the Cell Q™. The Cell Shuttle automates cell therapy manufacturing, and the Cell Q automates quality control (both in-process QC and release testing). Interconnected software systems enable the auto-generation of electronic batch records, thereby reducing human labor as well as possibilities for operator error. Cellares’ partners will benefit from unprecedented scale, cost-efficiency, and speed with the highest standards of quality and safety.
Additional IDMO Smart Factories are planned for the U.S. and overseas. Together, these facilities will allow Cellares to meet its commitment to accelerate access to life-saving cell therapies by transforming manufacturing and enabling cell therapy developers to meet the total global patient demand for these products.
Cellares' CEO, Fabian Gerlinghaus, expressed his enthusiasm for this milestone, stating, "Bringing our first cGMP Cell Shuttle online in New Jersey is a major step for Cellares and our partners. This facility showcases our innovative approach and aligns with our mission to provide life-saving cell therapies to patients in need, faster and more efficiently than ever."
For more information about Cellares, please visit www.cellares.com.